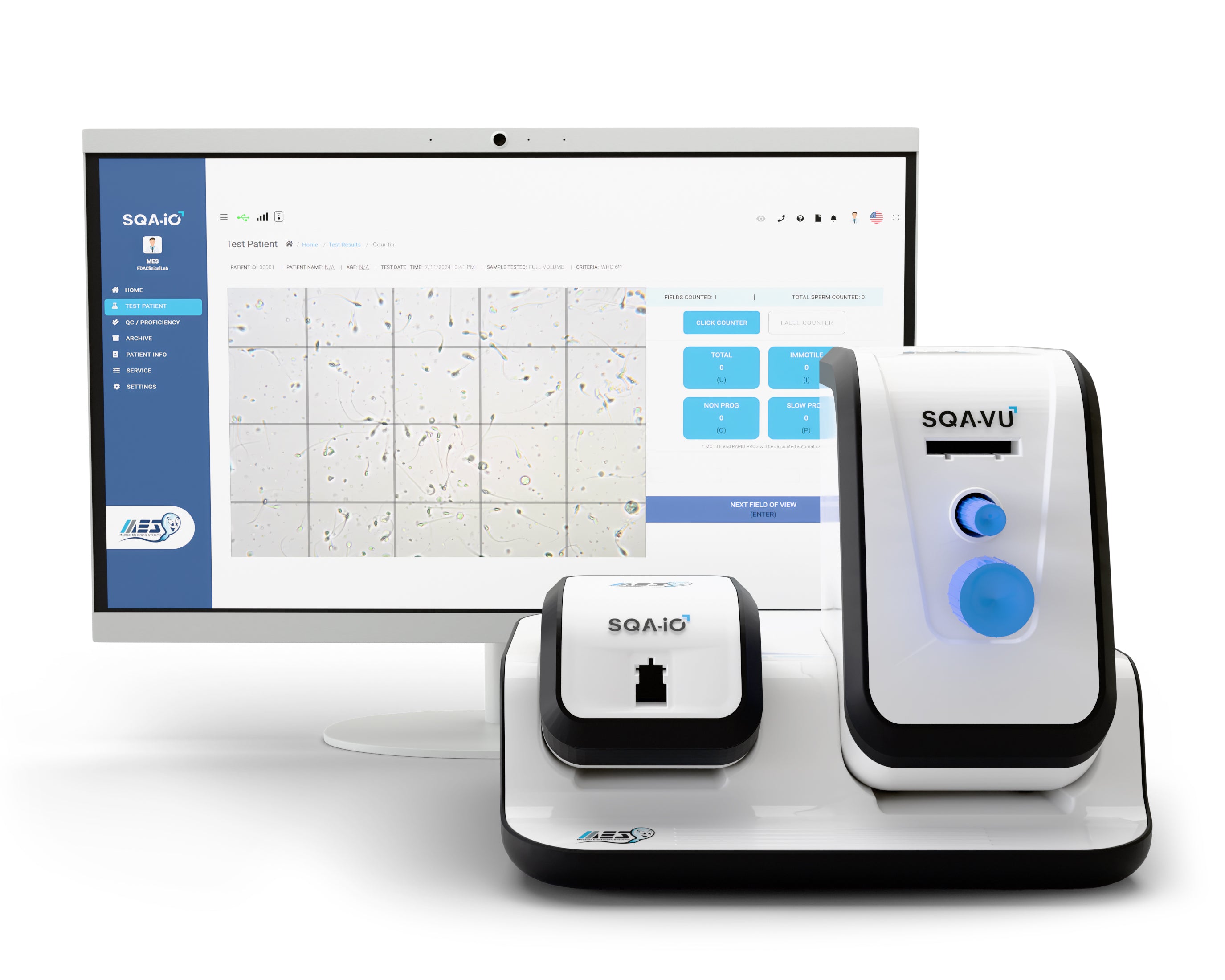
The Proficiency, Training, and Validation Kit for SQA systems ensures accurate validation of precision, lower limit detection, and reportable range in compliance with CLIA Method Validation Regulations.
- Offers a comprehensive solution for bringing new SQA instruments online quickly, helping to establish accurate baseline performance data for optimal use from day one
- Gives labs the supplemental documentation to pass inspections and demonstrate their adherence to industry standards
- Serves as both a training and proficiency tool, ensuring that lab staff are familiar with system operations, proper technique, and are equipped with the knowledge needed for high-quality analysis

Kit Includes:
- 4 Bottles of Precision Beads
- 6 Bottles of Linearity Beads
- 20 Bottles of Accuracy Beads
- 35 Disposable Capillaries
- USB Flash Drive
Master Validation and Training with Confidence
This is an initial Validation Kit that is designed for verifying the performance of new or relocated SQA sperm quality analyzers, ensuring accurate calibration across multiple testing parameters, including accuracy, precision, and lower limit detection. This kit is crucial for establishing a solid foundation of validation when setting up a new system or after moving a machine, ensuring it meets all necessary CLIA Method Validation Regulations.
Frequently Asked Questions
This kit is designed to provide comprehensive training and validation for new SQA-V and SQA-Vision analyzers. It ensures that your team can confidently assess accuracy, precision, linearity, and lower limit detection, meeting CLIA standards and optimizing analyzer performance right from the start.
With pre-measured beads, detailed protocols, and a USB flash drive for guidance, the kit simplifies the validation and training process. Medical Electronic Systems also crunches your data at no additional cost, saving your lab valuable time while ensuring accurate and reliable results.
The kit not only validates your analyzer but also provides hands-on training for capillary loading, system operation, and reporting. By building expertise during the initial setup, it helps your team confidently integrate SQA analyzers into your lab’s workflow, ensuring long-term success.